Have you ever faced a production line shutdown because a crucial component was unexpectedly out of stock? Or dealt with a costly product recall because you couldn’t pinpoint which batches used a faulty raw material? Such nightmares highlight a simple truth: in manufacturing, inventory management and quality control are deeply interconnected.
Effective inventory management isn’t just about tracking stock levels – it’s about ensuring that every part, material, and product in your facility meets quality standards and is handled in a way that preserves that quality. In fact, inventory quality control in manufacturing can make the difference between consistent, high-quality output and costly mistakes that erode customer trust.
Inventory Management and Quality Control: A Critical Connection
Inventory management is often defined as the process of ordering, storing, and using a company’s materials and products. Quality control, on the other hand, focuses on ensuring that those materials and finished products meet specific standards and specifications. In a manufacturing environment, these two functions are two sides of the same coin. How you manage inventory directly impacts your ability to produce quality goods consistently.
Think of it this way: your finished products are the sum of all their parts. If you have the right parts and materials in the right condition at the right time, your production line can operate smoothly and turn out high-quality products. Inventory management ensures that happens. It involves not just tracking quantities, but also monitoring the condition, location, and usability of each stock item. Quality control relies on that foundation – it checks that each item and process meets quality standards. Without reliable inventory control, quality control inspectors might be looking at the wrong data or, worse, missing critical issues like expired materials or incorrect components being used.
Effective inventory management streamlines processes and prevents problems at the source, thereby improving quality control. For example, if all components are stored in organized, labeled locations, and inventory software is updated in real time, workers on the line can quickly find the correct part and won’t accidentally use the wrong material. This reduces errors and defects. Additionally, keeping optimal inventory levels (not too much, not too little) avoids situations where parts sit for too long and potentially degrade, or where sudden shortages force you to use subpar substitute materials. In short, when inventory is well-managed, production has exactly what it needs to maintain quality – nothing more, nothing less.
Quality control also feeds back into inventory management. Every time a defect or non-conformance is found, it often ties back to a specific batch of material or a step in the process. With proper inventory tracking, you can identify which batch of raw materials was faulty or which machine produced a defective part, and then isolate those items in your stock. This prevents further use of bad components and triggers corrective actions like quarantine or re-inspection. Conversely, without good inventory records, a quality issue can spiral – you might end up scrapping a lot of good product or recalling more units than necessary simply because you can’t trace which items were affected.
The Impact of Poor Inventory Management on Quality

To appreciate the importance of inventory management for quality, it helps to consider what can go wrong when inventory practices are lacking. Unfortunately, many manufacturers have learned the hard way that poor inventory management can lead directly to quality issues. Here are some common quality pitfalls that stem from inventory control failures:
Using the Wrong or Substandard Materials: If inventory records are inaccurate or parts aren’t stored in designated places, employees might grab the wrong item for production. In a complex product with many similar-looking components, one mislabeled bin can lead to a serious defect in the final product. Likewise, without proper vetting of inventory, defective or substandard raw materials could be mixed in with good stock. For example, imagine a batch of bolts from an unverified supplier ending up on the line due to lack of controls – the resulting product could fail quality tests or, worse, fail in the field.
Expired or Degraded Stock in Production: Poor inventory rotation and oversight can result in materials exceeding their shelf life or becoming damaged in storage. For instance, chemicals, pharmaceuticals, food ingredients, or even certain electronics components have expiration dates or special storage requirements. If these items aren’t tracked and rotated (e.g. using a FIFO – First-In, First-Out system), expired or spoiled materials might accidentally get used in production. The outcome is predictable: the quality and safety of the finished goods are compromised. A classic example is paint or adhesive that’s past its prime; it may cause a product to fall apart or not meet specifications.
Inconsistent Production and Downtime: Quality isn’t just about the product specs – it’s also about consistency. Poor inventory management can cause frequent production stops, rush orders, or process adjustments, all of which threaten consistency. If a machine must halt because a part is suddenly out of stock (even though your system said it was available), you might later scramble and use a different material or a different process to catch up. Such firefighting can introduce errors and variations. In contrast, a well-managed inventory ensures smooth, uninterrupted production runs, which helps every unit come out uniform and within tolerance. Unplanned substitutions or process changes due to missing inventory often lead to quality slips.
Inability to Trace and Contain Defects: This is a major one – when inventory tracking is sloppy, traceability becomes a nightmare. Suppose a customer finds a defect in your product that originated from a bad batch of raw material. If you have no lot tracking or traceability system, you might not know which products or production lots used that raw material. The result? You’re forced to recall a huge swath of products “just in case,” or you miss some affected units entirely. This shotgun approach to quality problems is incredibly costly and damaging to your reputation. On the other hand, if you can pinpoint exactly which lot of finished goods used the defective batch, you can isolate and deal with only those items. Poor inventory management deprives you of that precision, turning what could be a minor contained issue into a widespread quality crisis.
Higher Scrap and Waste: When inventory isn’t controlled, manufacturers often see higher rates of scrap, rework, and waste – all indicators of quality problems. Materials might be mishandled, get lost, or deteriorate in storage (for example, moisture-sensitive components left out too long). You may also overproduce or overstock items that end up expiring or becoming obsolete, essentially throwing money and effort away. Each of these issues not only hurts your bottom line but also points to lapses in maintaining quality (for instance, storing materials improperly or not using them in time). Robust inventory practices help minimize these forms of waste by ensuring materials are used while they’re good and stored under the right conditions.
Best Practices for Inventory Management that Support Quality Control
The good news is that manufacturers can implement proven best practices to align inventory control with quality assurance. By introducing some key processes and protocols, you’ll greatly reduce the chance of inventory-related quality issues. Below are some inventory management best practices specifically geared toward maintaining product quality and consistency:
- Establish Clear Organization and Labeling: It all starts with a well-organized stockroom or warehouse. Every item, from raw materials to finished goods, should have a designated location that is clearly labeled. Use part numbers, descriptions, or even images on labels to avoid confusion. When everything has a “home” and is properly identified, workers are far less likely to pick the wrong component or mix up similar items. Organization also means separating non-conforming or expired stock (more on that shortly) so it’s never accidentally used. A place for everything and everything in its place – this motto underpins both inventory accuracy and quality.
- Implement First-In, First-Out (FIFO) Rotation: For any materials that can expire, degrade, or become obsolete, FIFO is critical. This means the oldest received stock is used first in production. By rotating inventory such that earlier lots get issued before newer lots, you prevent items from sitting untouched until they go bad. FIFO is especially important in industries like food, pharmaceuticals, chemicals, and even electronics (think of solder paste or batteries). Even if items don’t have formal expiration dates, FIFO ensures a healthy turnover and minimizes the chance of hidden deterioration. Using fresh and within-spec materials is a fundamental quality requirement, and good inventory rotation guarantees that.
- Use Batch/Lot Tracking for Traceability: Assign lot numbers or batch codes to incoming materials and keep records of which lots go into which production runs. This practice, known as batch tracking or lot traceability, is a quality-control lifesaver. If a defect or contamination is discovered, you can trace back to the specific lot of material or component that’s responsible. For example, if you receive raw material in lots A, B, and C, and later find that products made with lot B have issues, you can quickly isolate all items made with lot B. This ability to “trace products back to their origin” and isolate defective batches not only improves quality control (by addressing the root cause) but also helps maintain compliance with regulatory standards in regulated industries. The key is to record lot numbers at every inventory touchpoint: receiving, during production (which lot went into which work order), and in finished goods. Modern inventory systems or even a simple spreadsheet can help tie this information together.
- Conduct Regular Inventory Audits and Inspections: Don’t just trust the numbers in the system – verify them periodically. Regular cycle counts or full inventory audits ensure that the physical inventory matches the records. How does this help quality? First, it catches discrepancies that might indicate theft, loss, or misplacement (for instance, if an expensive part is missing, you’ll investigate and prevent a potential quality issue from using a “ghost” item that isn’t actually there). Second, audits can include quality spot-checks: while counting items, you might inspect their condition (packaging intact, no damage, within expiration date, etc.). This is an opportunity to find any stored inventory that has quietly become unsellable or unusable. By finding and removing bad stock proactively, you ensure that only good-quality materials stay in the available inventory. Audits also reinforce discipline in inventory handling, which in turn upholds quality – if people know inventory is routinely checked, they handle items more carefully and follow procedures.
- Set Up Quality Checks at Receiving: The inventory journey begins when materials arrive at your facility. Implement an Incoming Quality Control (IQC) process for all deliveries. This means that when raw materials, components, or parts are received, they are not just counted into inventory – they are also inspected for quality criteria. This could involve visual checks for damage, verification against specifications or certificates, and even functional testing for critical parts. Only materials that pass inspection should be “accepted” into your usable inventory. Materials that fail should be labeled and segregated (e.g., in a quarantine area) so they don’t accidentally slip into production. By catching problems at the door, you prevent defective inputs from ever entering your manufacturing process. Additionally, capturing data at receiving – such as lot numbers, supplier info, and inspection results – builds a traceability record that is invaluable if issues arise later. Optimizing inventory at the point of entry ensures you’re starting with quality materials from the get-go.
- Maintain Proper Storage Conditions: Inventory management for quality isn’t only about counts and locations; it’s also about how items are stored. Many materials have specific storage requirements (temperature, humidity, cleanliness, etc.) that, if not met, can degrade quality. For example, certain adhesives or paints might need climate-controlled storage, and electronic components often require protection from static or moisture. Ensure your storage meets these needs: use climate control where necessary, implement First-In First-Out for sensitive items, and use appropriate containers or packaging. Regularly monitor storage conditions and the state of stored goods. Something as simple as a roof leak in the warehouse or a malfunctioning freezer can ruin inventory quality if left unnoticed. Good inventory management includes safeguarding materials while they are in storage, which in turn preserves the integrity of those materials for when they’re used in production.
- Establish Clear Procedures and Train Staff: Even the best system can fail if the people using it are not well-trained or disciplined. Develop standard operating procedures (SOPs) for all inventory-related activities – receiving, storing, picking for production, issuing materials, handling rejects, etc. These procedures should emphasize accuracy and quality at each step (for instance, “always scan the barcode when picking an item to ensure you have the correct part” or “if any part looks damaged or questionable, do not use it and report it to a supervisor”). Then, train your team on these procedures and the reasons behind them. When operators and warehouse staff understand that following inventory protocol directly impacts product quality and customer safety, they are more likely to comply. Make it a part of your company culture that inventory management is everyone’s responsibility and is a key part of delivering quality products. Regular refreshers, audits, and even incentives for inventory accuracy can help sustain this culture. Remember, humans are a critical component of the inventory quality loop – well-trained people will catch issues that systems might miss and will handle inventory with the care that quality products demand.
- Integrate Inventory Management with Quality Assurance Processes: Finally, ensure that your quality team and inventory/production team work in tandem. Quality control plans should reference inventory controls, and vice versa. For example, if quality control requires a sample check of finished goods, ensure inventory records reflect those samples (so they aren’t later counted as sellable stock by mistake). If a certain raw material lot is failing quality tests, inventory management should flag and hold all items from that lot. Basically, build bridges between your inventory system and quality system. In practical terms, this could mean using software that links inventory data with quality data (like non-conformance reports or test results), or simply having cross-functional meetings where inventory discrepancies and quality issues are discussed together. The goal is to catch any quality issues that have inventory implications (and vice versa) early and deal with them holistically.
By implementing these best practices, manufacturers create a robust inventory environment where everything is documented, traceable, and controlled with quality in mind. It transforms inventory management from a mere logistical task into a powerful tool for prevention – preventing errors, defects, and compliance issues before they happen.
Leveraging Technology for Better Inventory Quality Control

In the modern manufacturing landscape, technology is your ally in marrying inventory management with quality control. Manual methods (like pen-and-paper logs or spreadsheets updated by memory) are not only inefficient, but also prone to error – and as we’ve seen, errors in inventory can quickly become quality problems. Fortunately, today’s inventory management technologies are designed to enhance accuracy, visibility, and responsiveness, all of which directly benefit quality control efforts. Here are some ways technology can boost inventory quality control:
- Barcode Systems for Accuracy: Barcodes are a staple of inventory management for good reason. By tagging every item or material with a barcode (or QR code) and using scanners, you ensure that each movement of inventory is recorded correctly. When a technician scans a component before using it in production, the system can verify it’s the right part, from the right lot, and even check if it’s within its expiration date if that data is recorded. This greatly reduces human error – no more relying on someone reading tiny part numbers or scribbling down the wrong info. Barcode scanning at receiving helps catch quantity errors or mis-shipments from suppliers; scanning at issue and return keeps your inventory records accurate in real time. The result is a high fidelity inventory database that quality control can rely on. It also speeds up processes (faster than manual entry), which means quality inspections and operations can proceed without bottlenecks. In short, a well-implemented barcode system ensures the right materials are in the right place at the right time, and you have a trustworthy log of every inventory transaction.
- RFID for Real-Time Tracking: Radio Frequency Identification (RFID) takes automated tracking a step further by allowing items to be identified and tracked without direct line-of-sight scanning. In manufacturing, RFID tags attached to materials, parts, or even finished products enable real-time monitoring and traceability throughout the facility. For instance, as RFID-tagged components move from the warehouse to the production floor, and eventually into finished goods inventory, fixed readers at doorways or workstations can automatically log those movements. The benefit for quality control is immense: you get an up-to-the-minute picture of where any given lot or item is, and you can quickly pinpoint discrepancies. RFID can also support quality by monitoring process parameters – some advanced tags can even record environmental data or sense shocks (useful to see if a sensitive component was dropped or exposed to out-of-range temperatures). While implementing RFID has an upfront cost, it reduces manual data entry errors and can catch issues like items being in the wrong location or unauthorized changes in inventory. Additionally, because RFID systems can handle bulk reading (scanning many tags at once), doing inventory audits or locating all items from a suspect batch becomes much faster, saving valuable time during a quality investigation or recall.
- Inventory Management Software (IMS/ERP systems): At the heart of leveraging technology is using a robust inventory management software or an ERP (Enterprise Resource Planning) system with inventory modules. These digital platforms act as the central brain for all inventory data and transactions. By using an inventory software, you gain real-time visibility into stock levels, locations, and movements. This visibility means no more guesswork – production planners and quality managers can see exactly how much of a material is on hand, which lot is allocated to which production job, and whether any quality hold is in place. Modern inventory software often includes features like automatic reordering alerts (to avoid stockouts that cause quality shortcuts), integrated bill of materials (BOM) management (ensuring the correct parts are kitted for each product build), and quality status flags (e.g., you can mark a batch as “QC hold” so it can’t be used until cleared). Many systems also allow you to attach documents and certificates to inventory lots, so quality certificates or test results are just a click away. A huge advantage is data analysis: these systems can generate reports on inventory accuracy, supplier quality (e.g., how often lots from Supplier X fail inspection), and even track metrics like time in storage vs. defect rates. By analyzing such data, a manufacturer can make informed decisions, such as changing a supplier who often provides bad material, or adjusting storage practices if a pattern shows that items stored over 6 months tend to fail more.
- Quality Control Integration: Some advanced inventory systems integrate quality control workflows directly. For example, when a lot is received, the system can prompt an incoming quality inspection checklist on the spot. Until that inspection is completed and passed, the inventory might remain in a “pending” status in the software. Similarly, if a quality issue is detected in production, a good system can automatically adjust available inventory (perhaps by placing remaining material from that lot on hold). By integrating these workflows, you remove the gaps between inventory and quality data. Everyone – from warehouse staff to quality engineers to managers – can see a unified picture. During audits (internal or external), having an integrated system means you can quickly retrieve evidence of quality control steps for each inventory lot and finished product. This not only saves time but also boosts confidence in your operations’ integrity.
- Automation and Industry 4.0 Technologies: Beyond barcodes and software, the broader Industry 4.0 movement brings IoT sensors, automation, and AI into play. (We’ll avoid diving into “IoT” buzzwords too much, but it’s worth mentioning how some of these emerging technologies contribute without naming them outright.) For example, automated storage and retrieval systems (AS/RS) in warehouses can ensure items are stored properly and picked correctly, reducing handling damage (which maintains quality) and virtually eliminating picking errors. Sensors can monitor conditions like temperature and humidity in real time and alert you if a threshold is breached, so you can take action before materials are ruined. Artificial intelligence can analyze inventory and production data to forecast demand more accurately, which prevents overproduction or long storage times that might affect quality. AI can also help identify patterns, such as correlating a certain supplier’s materials with higher defect rates, so you can proactively address it. Essentially, automation and smart technology add an extra layer of vigilance – they can work 24/7 to maintain the environment and accuracy of your inventory, allowing your human team to focus on higher-level quality improvement tasks.
Incorporating these technologies doesn’t mean you abandon the human element or basic best practices – rather, technology augments your team’s abilities. It enforces discipline (e.g., you can’t skip scanning a barcode if the system requires it to proceed), provides instantaneous information, and reduces the mundane workload (like endless manual counting and writing) so that people can concentrate on problem-solving and process improvement. When evaluating technology solutions, always consider how they will specifically help maintain or improve quality. For instance, does the software allow for tracking of quality status? Can it record lot numbers and test results? Does it provide audit trails for every inventory move? By asking these questions, you ensure that your investment in technology directly supports your quality control mission.
Ensuring Traceability and Compliance through Inventory Management

For manufacturers in industries like food & beverage, pharmaceuticals, automotive, aerospace, or medical devices, quality control isn’t just about customer satisfaction – it’s a legal and regulatory requirement. Even in less-regulated industries, being able to trace your products and components is a mark of a well-run operation and is invaluable when something goes wrong. Inventory management plays a central role in achieving the traceability and compliance that standards and regulators demand.
Traceability means being able to track every ingredient, part, or product from its origin all the way to the end customer, and vice versa (from any final product back to the specific batches of inputs). Achieving this requires diligent inventory records at each step of the manufacturing process:
- Lot Tracking from Raw Materials to Finished Goods: As mentioned earlier, assigning lot or batch numbers to incoming materials is step one. The next step is to carry those identifiers forward. When you issue materials to a production order, record which lot numbers were used for which order. When that order produces finished goods, those goods should inherit some record of the input lots (and have their own batch number if you produce in batches). This way, if a supplier informs you that raw material lot #123 is contaminated or defective, you can quickly query your records and find exactly which finished products used lot #123. You might find, for example, that only Product Batch X and Y (out of many) contain that material – so only those batches need to be put on hold or recalled. This targeted recall ability is hugely beneficial. It prevents the nightmare scenario of recalling everything “just in case,” which is expensive and can tarnish your brand unnecessarily. Moreover, if you’re audited by a regulatory agency or a big client, you can demonstrate with confidence, “For any given product unit, we can trace all its components back to their source lots and suppliers.” This level of traceability is often mandatory for certifications like ISO 9001, ISO 13485 (medical devices), or compliance with FDA regulations, to name a few.
- Real-Time Record Keeping and Document Management: Compliance often requires proper documentation – certificates of analysis, material safety data sheets, conformance certificates, etc., must be linked to the materials and products. A good inventory management process will include capturing these documents at receiving and storing them in an accessible way (many inventory or ERP systems let you attach files to inventory records). For instance, a Certificate of Conformance from a steel supplier that certifies the chemical composition of a batch of steel can be scanned and attached to that batch’s record in your system. Later, if a customer or auditor asks for proof that you used certified materials, you can retrieve it in minutes. Additionally, maintaining calibration records for any tools or equipment (as inventory items) is part of quality compliance in some industries – you might track gauges or jigs as inventory with serial numbers and log their calibration due dates, ensuring that only calibrated equipment is used in production. Inventory management and document management go hand-in-hand for compliance: every item is not just a physical thing but carries with it a trail of data and paperwork that proves it meets standards.
- Preventing and Handling Recalls Efficiently: Even with the best controls, sometimes a recall or field corrective action is necessary (due to a safety issue, for example). When that happens, a company with strong inventory traceability can react swiftly and effectively. Your inventory system should allow you to search by lot number or product serial number and find all locations and products where that item resides. For example, if you must recall a particular batch of product, your records will tell you exactly how many units were produced, their lot numbers, and which customers or distribution centers received them. This means you can contact only those affected parties. It also means if some of that batch is still in your finished goods warehouse, you can quarantine it immediately to prevent any further shipments. During a recall situation, time and accuracy are critical – regulators will ask for detailed reports, and customers expect quick communication. Good inventory management underpins both: you’ll spend less time scrambling to gather information and more time executing the recall and communicating clearly. It’s worth noting that demonstrating control during a recall (having accurate counts, knowing exactly where suspect product is, etc.) can mitigate regulatory penalties or liability, because it shows you were responsible and proactive.
- Compliance Audits and Inventory Records: Regulatory bodies and certification auditors often inspect inventory-related practices. They may want to see calibration stickers on equipment, lot number logs, FIFO implementation on the warehouse floor, or how non-conforming material is segregated. If your inventory management is solid, these will be easy to showcase. For example, an auditor might pick a random finished product and ask you to trace its components – your inventory system should let you do that on the spot, drilling down into the product’s build history and pulling up each component’s origin and any inspection records. Auditors also appreciate seeing that you have controls to prevent mistakes, such as barcode systems or system alerts for expired stock. Many industries require proof of an inventory control plan as part of quality management. By integrating inventory management into your overall quality management system (QMS), you can meet sections of standards that deal with things like identification and traceability, preservation of product, and control of monitoring and measuring equipment (all typical clauses in ISO 9001, for example).
- Maintaining Only “Good” Inventory for Use: Compliance and quality assurance often mean having procedures to ensure that only approved, inspected materials are used, and that any non-conforming items are quickly removed from circulation. Inventory management supports this by using status labels or separate storage. For instance, you might have your inventory system set up with statuses like “Accepted,” “Quarantine,” “Rejected,” “On Hold,” etc. A new lot of raw material might be in “Quarantine” until incoming inspection approves it – at which point its status is changed to “Accepted” and it becomes available for production. Similarly, if a finished product batch is awaiting final quality test results, it might be marked as “On Hold” in the system and perhaps isolated in a specific physical area. This way, there’s no confusion: anyone can see that these items aren’t cleared for use or shipment yet. Visual cues in the warehouse (like red tags on rejected items) combined with system controls create a fail-safe that ensures off-spec products don’t accidentally get mixed in with good ones. This process is vital for compliance, as it demonstrates control over non-conforming outputs and adherence to procedures for disposition of such items (whether rework, scrap, or return to vendor).
In summary, robust inventory management gives manufacturers the control and visibility needed to meet quality and compliance obligations. It turns traceability from a daunting task into a byproduct of everyday operations – because if you’re consistently capturing lot data, movements, and quality status in your inventory system, then compiling that information for an audit or recall is straightforward. Companies that invest in these practices not only avoid regulatory trouble but often find that it opens up new business opportunities too (for example, being able to prove traceability can win you contracts in demanding sectors, as clients know you’re a lower risk supplier). In today’s manufacturing world, traceability and quality go hand in hand, and both begin with diligent inventory management.
How CyberStockroom Supports Inventory Quality Control
To bring these concepts to life, let’s look at how an inventory management tool like CyberStockroom can help manufacturers maintain high quality standards and keep inventory under control. CyberStockroom is a unique inventory management software known for its visual inventory map interface, which can be especially useful for operations managers who want a clear, intuitive view of their stock across multiple locations. Here are several ways CyberStockroom’s features and approach align with the quality control and compliance needs of manufacturers:
Visual Inventory Map for Full Visibility
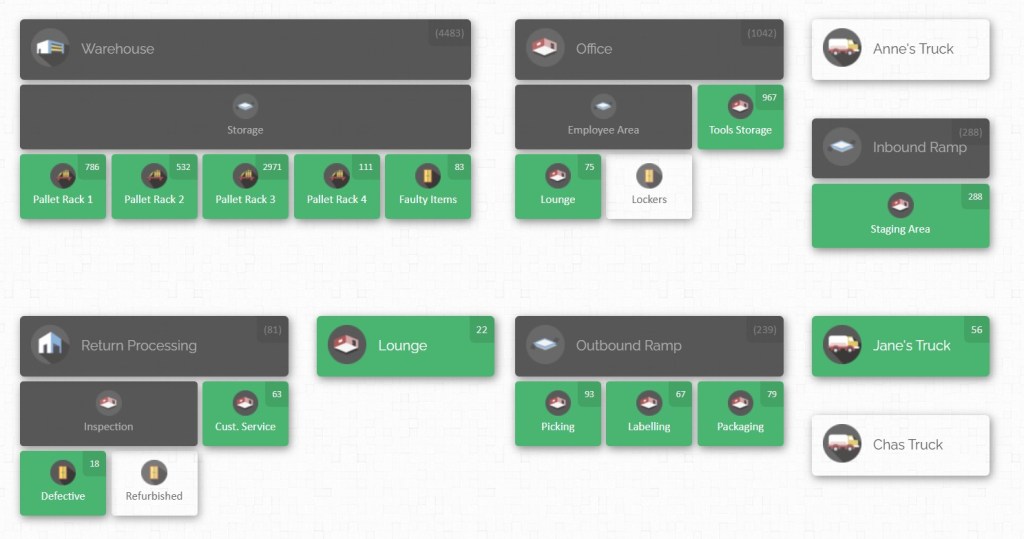
CyberStockroom allows you to create a customized map of your business – whether that’s a single factory floor or multiple warehouses and storage areas. This bird’s-eye view means at any given moment, you can see where all your inventory is located and how much is at each place.
From a quality perspective, this helps ensure nothing is “lost” in some corner of the plant. You can quickly drill down into a location on the map (say, the Quarantine cage or the Finished Goods cold room) and view its contents. Such visibility makes it easier to enforce storage rules (you’ll notice if something is stored in the wrong area) and to respond when a quality issue arises. The map interface is also a communication tool – it’s much easier for teams to discuss inventory and quality issues when looking at a visual layout, improving collaboration between inventory managers and quality teams.
Real-Time Updates and Easy Item Movement
In CyberStockroom, when inventory moves or changes (for example, you transfer raw materials from the main warehouse to the production floor storage), those changes can be updated in real time on the map. Using a drag-and-drop interface, users can move items between locations on the map, which instantly adjusts the inventory records. For quality control, this immediacy is valuable. It means the data everyone sees is up-to-date, reducing the risk of using an item that was just relocated or depleted.
Also, CyberStockroom’s ease of performing cycle counts and adjustments (thanks to the drag-and-drop and map clicking features) encourages frequent inventory verification. Regular cycle counting, as we covered, helps catch errors and quality issues early. CyberStockroom essentially makes the task less daunting and more accessible to the team, so it’s more likely to get done. The software’s focus on simplicity and clarity helps your staff stick to inventory procedures, which in turn supports quality – after all, a system only helps if people actually use it consistently!
Barcode Scanning Integration
CyberStockroom supports barcode technology, allowing you to scan items into and out of inventory. This aligns perfectly with quality best practices. With barcoding in CyberStockroom, you can assign barcodes to each product or part in your catalog, and then scan items during receiving, transfers, or usage. Scanning greatly reduces data entry mistakes – the system will alert you if you try to add an item that doesn’t exist in the catalog or if you scan an item into the wrong location (depending on how you set it up).
When receiving goods, scanning their barcodes (which could encode lot numbers or item IDs) ensures you log exactly what was delivered. During production, scanning components as you consume them means you always know what lot went into a work order. And for shipping finished goods, scanning verifies you’re sending the right product out. All these scanned confirmations build a reliable audit trail. CyberStockroom’s use of barcodes thus helps maintain the integrity of inventory data, which quality control relies on. It also speeds up operations – quick scans vs. typing – meaning quality checks and inventory logging happen with minimal friction.
Custom Fields for Quality Tracking
Every manufacturing operation has its own critical data points. CyberStockroom allows customization, meaning you can add custom fields to inventory items. This is incredibly useful for incorporating quality-related information into your inventory system. You could record a “Certificate #” or “Heat Number” for materials that come with specific quality documents. By capturing this data in CyberStockroom, the inventory map becomes more than just a count – it becomes a rich source of quality info.
Having these details at your fingertips allows managers to make quick decisions. It also means during audits or investigations, you don’t have to cross-reference multiple systems or paper files – the inventory system already holds the key facts. CyberStockroom essentially can serve as a light-weight quality management log in addition to tracking quantities.
Loss Prevention and Traceability Features
CyberStockroom includes features aimed at loss & theft prevention and provides an activity history for inventory movements. While on the surface this is about security and cost (knowing if something “went missing”), it overlaps with quality by maintaining traceability. Every time an item is moved, checked out, or adjusted, the system can log who did it and when. This means accountability – if a part bypassed a procedure or got used without authorization, you have a record.

For example, if an operator takes out a replacement part for a machine (MRO inventory) via the system, there’s a trail. If later that part is linked to a problem, you know when it was installed and from which stock it came. CyberStockroom’s approach of tracking user actions and offering permission controls ensures that only authorized personnel can, say, move inventory from Quarantine to Available stock (reflecting that QA approved it). These controls and logs act as checks and balances. They make it much harder for something to be mistakenly or intentionally done outside of established protocol without it being noticed.
Cloud-Based Convenience and Collaboration
CyberStockroom is a cloud-based platform, which means your team can access the inventory data from any device with an internet connection. Being cloud-based also implies that updates roll out to everyone instantly; there’s one version of truth. This fosters collaboration – production, warehouse, and quality teams are literally on the same page (or rather, the same map). For instance, when quality puts a hold on a material, they can immediately update it in CyberStockroom, and the warehouse team across the plant will see that reflected and know not to use that material. This kind of real-time synchronization prevents miscommunication and errors, especially in fast-paced manufacturing settings.

In summary, CyberStockroom’s features are aligned with the needs we identified earlier in this blog. It helps maintain organization and visibility, supports FIFO and traceability with custom data like expiration dates and lot info, encourages regular audits with easy counting features, and integrates scanning technology to minimize mistakes. It’s also user-friendly and visual, which means your team is more likely to use it correctly (reducing the learning curve often associated with complex inventory software). By using a tool like CyberStockroom, manufacturers can simplify the implementation of many best practices we discussed – the software becomes a backbone that keeps inventory and quality control connected.
Of course, CyberStockroom is one tool among many, and each company should evaluate what features are most important for their specific processes. The key takeaway is that leveraging the right inventory management software can greatly reduce the manual burden of traceability and quality compliance, allowing you to focus more on making great products rather than fighting fires in the stockroom.

Building a Quality-Focused Inventory Culture
Implementing processes and deploying technology are crucial steps, but sustaining high standards in inventory management and quality control also requires the right culture and mindset in your organization. For general operations managers, it’s important to foster an environment where the workforce understands the value of inventory discipline and how it ties into quality outcomes. Here’s how you can build and maintain a quality-focused inventory culture:
- Leadership Emphasis: Make it clear from the top-down that inventory accuracy and quality are non-negotiable priorities. When management regularly asks about inventory accuracy metrics or participates in spot checks, it sends a strong message. Tie inventory management goals to performance reviews or KPIs for teams. For example, you might track “inventory accuracy rate” or “number of quality incidents due to inventory errors” as metrics that teams work to improve. Celebrate successes – if the warehouse team achieves 98% inventory accuracy or helps catch a potential quality issue before it escalated, recognize their contribution. When employees see leadership cares about these details, they will mirror that care in daily tasks.
- Ongoing Training and Empowerment: Initial training is important, but things change – new products, new employees, or new systems. Make training on inventory procedures and quality standards a continuous effort. Conduct refreshers or toolbox talks on topics like proper material handling, the importance of lot tracking, or how to use new features in your inventory software. Encourage employees to speak up if they notice an inventory discrepancy or a potential quality issue. Often, it’s the people on the floor who spot the broken crate, the leaking drum, or the mis-tagged component first. Create a culture where raising a concern is welcomed and rewarded, not ignored or punished. This empowerment can be formalized by giving certain team members roles like “Inventory Quality Champion” who liaise with both the quality department and warehouse, ensuring that communication flows and small issues get addressed early.
- Cross-Department Collaboration: Quality control should not operate in a silo separate from inventory/logistics. Create regular touchpoints between the quality assurance team and the inventory/warehouse team. This could be as simple as a weekly meeting to review any non-conforming material reports, discuss upcoming new materials that may need special handling, or review any near-misses (e.g., “we almost used the wrong resin in production last Tuesday, what happened and how do we prevent it?”). By collaborating, each side understands the challenges and realities of the other. The quality team can help craft better inventory handling procedures (because they know the critical control points), and the inventory team can give feedback on what’s practical or suggest improvements to quality inspection flows. This partnership reinforces the idea that everyone is collectively responsible for maintaining quality through effective inventory control.
- Continuous Improvement Mindset: Just like any other aspect of manufacturing, inventory management and its impact on quality should be part of your continuous improvement program (e.g., Kaizen if you practice lean manufacturing). Analyze data from your inventory and quality systems to find patterns and areas for improvement. Maybe you notice that a particular storage area has more issues (items getting lost or damaged) – that could indicate a need for reorganization or better shelving. Or perhaps a certain type of part often shows up in the wrong location – maybe its labeling or packaging is confusing and needs enhancement. Use root cause analysis for inventory inaccuracies that led to quality problems: was it a training issue, a system issue, or a simple mistake? Then implement corrective actions just as you would for a production defect. Engage the front-line workers in these improvements; often they have great ideas for how to make things smoother. For example, a worker might suggest color-coded labels for different product families to reduce mix-ups – a simple fix that management might not have thought of. By continuously refining your inventory processes with quality in mind, you also keep the team engaged and prevent complacency.
- Aligning Inventory Management with Overall Quality Goals: Many manufacturing companies have overarching quality goals such as reducing defect rates, achieving zero customer complaints, or obtaining a certain quality certification. Make sure your inventory management strategy is explicitly linked to those goals. If your goal is to reduce defects by X%, consider how inventory errors currently contribute to defects and set sub-goals to tackle that. If a goal is to get ISO 9001 certified, ensure your inventory procedures meet the traceability and documentation requirements of that standard, and do a mock audit focusing on inventory. When employees see that strong inventory control is actually a means to achieving big-picture success (like winning an industry award or landing a huge customer contract due to your quality reputation), it adds motivation. People often take pride in knowing their diligence in managing inventory isn’t just pushing boxes, but is directly contributing to the company’s excellence and customer satisfaction.
In a truly quality-focused inventory culture, attention to detail becomes second nature. The stockroom clerk understands that by double-checking that lot number, they might be preventing a future recall. The machine operator knows that by returning unused parts to the proper bin and updating the system, they’re helping the next shift and keeping records straight. When everyone internalizes that mindset, you gain not just fewer errors, but a more engaged workforce that acts as the first line of defense for quality control.
Conclusion: Inventory Management as the Foundation of Quality
Manufacturing high-quality products consistently is like building a sturdy structure – it requires a solid foundation. Inventory management is a core part of that foundation. Without controlled inventory, even the most sophisticated production processes or rigorous final inspections can falter, because the inputs and information they rely on are shaky. On the other hand, when inventory is well-managed – organized, accurate, traceable, and integrated with quality processes – it supports everything above it. Production lines get the right materials at the right time, quality checks occur at the right points, and any issues can be traced and resolved with minimal disruption.

We’ve seen how practical steps like FIFO rotation, lot tracking, and regular audits directly prevent quality problems. We’ve highlighted the value of technologies like barcoding and inventory mapping in enhancing both efficiency and quality assurance. And we discussed how CyberStockroom’s visual inventory management tool is one example of making these practices easier and more transparent, especially for operations managers who need a big-picture view as well as detailed control.
For manufacturers aiming for excellence in quality and compliance, focusing on inventory management yields significant returns. It reduces waste and scrap, prevents costly mistakes, ensures compliance with regulations through proper records, and ultimately leads to higher customer satisfaction because the products that leave your facility are as intended – no missing pieces, no subpar materials hidden inside, and consistent performance out in the field.
Perhaps just as importantly, good inventory management saves money and builds a more resilient operation. Fewer surprises in inventory mean fewer emergency expediting fees, less overtime spent fixing errors, and less risk of expensive recalls or reputation damage. It also gives you agility; if there is a problem, you can respond confidently and precisely, which is the hallmark of a mature, quality-driven organization.
In conclusion, the role of inventory management in quality control for manufacturers cannot be overstated. It is the glue that connects supply chain, production, and quality assurance into one coherent system. By investing time and resources into improving inventory processes – and by cultivating a culture that values accuracy and accountability – manufacturers set themselves up for long-term success. High quality isn’t achieved by quality control inspectors alone; it’s achieved by smart systems and informed people working together, starting from the moment materials enter your door to the moment finished goods ship out. And that journey, as we’ve detailed, is guided at every step by effective inventory management.
So, whether you’re troubleshooting recurring production issues or preparing for your next compliance audit, take a look at your inventory management practices. Strengthening them could be the key to unlocking better quality, stronger compliance, and greater peace of mind in your manufacturing operations. Quality products begin with quality practices – and managing your inventory is one of the most impactful quality practices of all.





Leave a comment